
Our Science
Responsive RNA control systems for precise control of gene therapies
Enhanced safety
Modular RNA control systems
Using our team’s expertise in mammalian synthetic biology and AI, Concinnity designs highly modular gene therapy control systems that are compatible with a broad range of therapies and existing technologies.
Our unique RNA-based systems enable the precise control of gene therapies even after dosing, conveying the ability for them to respond to and reduce their own side effects. This offers unprecedented precision and reliability that surpasses current capabilities.
Many other technologies use naturally occurring control systems as a starting point, but this limits functionality. Because our control systems are synthetic, we’re able to build them to respond to the input signals that gene therapy companies actually need.
Our technology has the potential to produce control systems responsive to any molecule, meaning the therapy will respond to biological or synthetic molecular signals. Our control systems are agnostic to the therapeutic type and can be used across many different therapy areas.
The benefits
Modular, multi-functional control systems
Our small RNA sequences can be used in mRNA therapeutics as well as heavily size constrained AAV delivery vectors.
The 3’ UTR location of our systems, a previously underutilised section of the gene cassette, offers improved modularity and compatibility.
Our highly modular control systems are compatible with other control technologies to further enhance safety.
Improved risk-to-benefit ratio enables the expansion from orphan diseases to larger indications, benefiting more patients.
Improved control systems
Responsive to the molecule of your choice
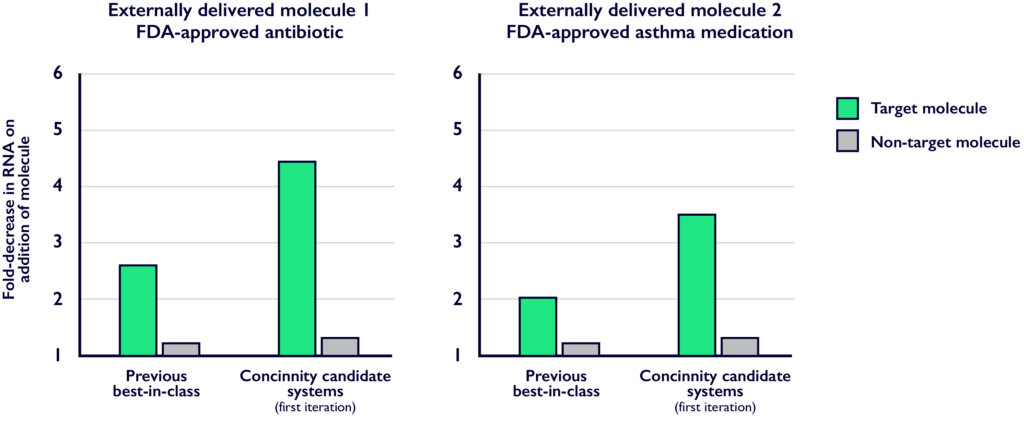
Concinnity has developed control systems that are responsive to internal or externally delivered molecules and show significant activity improvements over the previous best-in-class systems.
Using our proprietary platform, we are developing a portfolio of control systems responsive to a diverse range of input molecules and use our intelligent design cycle to improve functionality.
The platform
AI powered synthetic biology
Our platform uses a combination of high-throughput screening approaches and bespoke AI to learn from previous control system designs and predict improved variants. We give cells the ability to report on the functionality of potential control systems and use AI to understand this information.
This reduces time and costs enabling the rapid and predictable development of control systems responsive to almost any molecule. In contrast with traditional trial and error processes, our platform is expandable and scalable, allowing us to build not just one control system but many.

FAQS
People often ask us…
Find out more about our RNA control systems, how they work and how they could benefit your gene therapy pipeline.
Why do cell and gene therapies need to be controlled after administration to the patient?
Safety has been recognised as a key issue for gene therapies, with their use currently limited to orphan diseases. To expand their application, greater control of potential side effects is required.
For many conditions, an over- or under-production of a certain protein can cause two distinct diseases. To treat these diseases with gene therapies, the therapeutics need to be made responsive to their own activity to stay within safe bounds.
Why is Concinnity’s approach better than other safety mechanisms?
Many other technologies use naturally occurring control systems as a starting point, which limits functionality. Our unique RNA-based systems enable the precise control of gene therapies even after dosing, conveying the ability for them to respond to and reduce their own side effects. Because our control systems aren’t fully based on natural biological systems, we’re able to build control systems that respond to the inputs that gene therapy companies actually want.
What therapy areas will Concinnity’s systems work in?
Our control systems are agnostic to the therapeutic type and can be used across many different therapy areas. As they are RNA-based, they can be used in mRNA therapeutics, including gene editing, unlike traditional DNA-based control systems (e.g. synthetic promoters).
Our technology can play a leading role in the development of gene therapies targeting metabolic diseases or those diseases where an intracellular small molecule biomarker could be used to regulate the system in response to disease state.
By reducing the risk profile of gene therapies, our approach will expand the range of diseases that can be treated, benefitting a wider group of patients.
Do Concinnity’s systems work with other control mechanisms?
Our RNA sequences are very small and highly modular so can be incorporated alongside other methods of gene control. By locating our systems within a previously underutilised section of the gene cassette, the 3’ UTR, we offer improved modularity and compatibility.
Do your systems require additional genetic machinery?
Many gene control mechanisms require additional genetic machinery that either has to be co-delivered with the gene therapy or that is found in the cell. Our control systems are self-contained and do not rely on additional machinery.

Realise a new era in gene therapy
Partner with Concinnity to enhance the safety and expand the applications of your gene therapies.